- HOME
- Products
- HPLC Columns
- CAPCELL PAK MG Type
A column prepared with the greatest of care, concerning that even minute quantities of metallic impurities can greatly influence performance.
We provide sharp peaks to alleviate peak tailings in coordinate compounds susceptible to the influence of metals.
"While the peaks of other compounds present no problem, one particular compound exhibits a remarkable tailing for some unknown reason"
"Only one particular compound shows a peak rising gradually, making it difficult to define
a stabilized area"
Have you experienced such a problem?
In such a case, confirm the chemical structure of the sample. If there is any site easily to cause metallic coordination, it may due to metallic impurities.
Thanks to the polymer-coated base material and packing techhnology that take acount of the influence of metallic impurities, CAPCELL PAK C18 MG sharply elutes even compounds with highly coordinating properties like hinokitiol. Moreover, since the packing material has been designed to enhance surface polarity, it firmly retains even compounds with polarity sites like coordinate compounds.
Features
From polar to hydrophobic compounds
Among C18 columns, MG column presents an intermediate level of hydrophobicity and surface polarity. It is the recommeded colum for a wide range of substances, from polar to hydrophobic compounds.
For coordinate compounds in particular, peak tailing and absorption have been minimized to enable elution with sharp peaks.
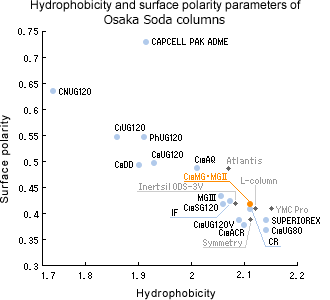
Coordinate compounds (hinokitiol) yet with a good peak profile
Thanks to the use of highly refined silica substrate and unique packing technology that minimized metallic impurity, the peaks of strong coordinate compounds like hinokitiol are eluted sharply. Because even trace amounts of metallic impurities greatly influence analysis results. Simply changing the column to MG will be your solution for absorption problem.
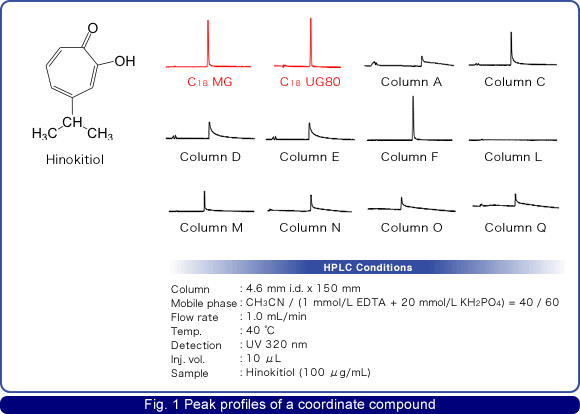
Good lot-to-lot reproducibility
Excellent lot-to-lot reproducibility has been achieved by Osaka Soda's silica gel manufacturing technology and column packing technology.
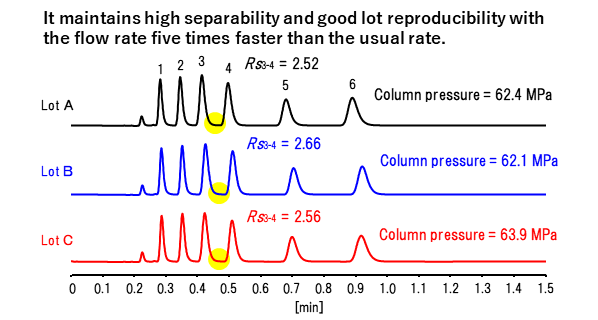
| Column | : | CAPCELL PAK C18 MG S2;2.0 mm i.d. x 50 mm |
|---|---|---|
| Mobile phase | : | 10 mmol/L HCOONH4/CH3OH = 95 / 5 |
| Flow rate | : | 1 mL/min |
| Temperature | : | 40℃ |
| Detection | : | UV 254nm |
| Inj. vol. | : | 1µL |
| Sample | : | 1. Uridine 2. 2'-Deoxyuridine 3. Guanosine 4. 2'-Deoxyguanosine 5. 2'-Aminoadenosine 6. Adenosine (50 µg/mL each) |
With a particle size of 2μ, ultra-high-speed analysis is possible due to excellent separation performance.
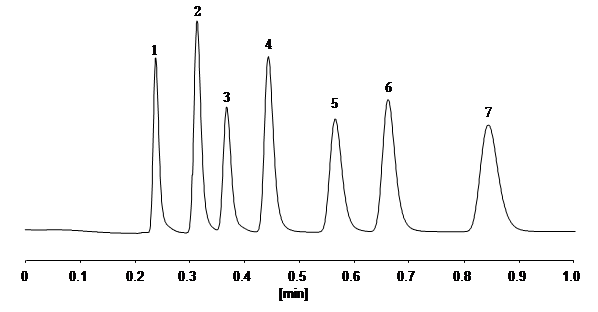
Seven types of sulfa drugs were analyzed at ultra-high speed using CAPCELL PAK C18 MG S2. The full analysis is done within 1 minute and all compounds achieve baseline separation.
fig2 UHPLC analysis
| Column | : | CAPCELL PAK C18 MG S2;2.0 mm i.d. x 50 mm |
|---|---|---|
| Mobile phase | : | 20 mmol /L Phosphate buffer (KH2PO4 : K 2HPO4 = 1 / 1 in molar ratio) / CH3OH = 80 / 20 |
| Flow rate | : | 900 µL/min |
| Temperature | : | 40℃ |
| Detection | : | UV 270nm |
| Inj. vol. | : | 1µL |
| Sample dissolved in | : | Mobile phase ※ 1 µg/mL = 1 ppm |
| Sample | : |
1. Sulfanilamide 2. Sulfamethizole 3. Sulfamerazine 4. Sulfapyridine 5. Sulfamethoxypyridazine 6. Sulfamethazine 7. Sulfaquinoxaline |
閉じる
Physical properties
| Functional group | Particle diameter (μm) |
Pore diameter (nm) |
Specific surface (m 2/g) |
C% | Density (µmol/m 2) |
pH range for use |
USP |
|---|---|---|---|---|---|---|---|
| Octadecyl group | 2 | 10 | 300 | 15 | 2.4 | 2~10 | L1 |
| Octadecyl group | 3 | 10 | 300 | 16 | 2.6 | 2~10 | L1 |
| Octadecyl group | 5 | 10 | 300 | 16 | 2.6 | 2~10 | L1 |


