- HOME
- Products
- HPLC Columns
- What is CAPCELL PAK?
High performance liquid chromatography (HPLC) is one of the most popular techniques of analytical chemistry. The performance of the analytical system largely depends on that of columns.
Osaka Soda provides an extensive lineup of products covering all polarity, from low to high, to meet the diversified needs of researchers.
To consistently obtain stable analytical results from any lot anywhere in the world, CAPCELL PAK is subject to thorough quality control at each production process from choice of starting materials to shipping.
|
| Characteristics of packing material by support type |
Silica packing materials, represented by octadecylsilyl (ODS-) silica and octylsilyl silica, have numerous features such as excellent pressure resistance and solvent resistance, high separation performance, wide sample range, and low column cost.
However, with the progress in studies of proteins, peptides, and other components and the research of pharmaceutical metabolism accompanying the recent development of biotechnology, several problems have surfaced, such as peak tailing and recovery deterioration due to the irreversible adsorption of components, especially basic compounds.
After ODS bonding, non-reacted silanol groups (Si-OH) are generally processed (end capped) using a trimethylsilyl agent to resolve the adsorption problem and improve durability. Even after the end capping process, however, it is known that C18 groups are hydolyzed in basic environments, and that silica itself dissolved into basic liquid. Therefore, conventional ODS columns' mobile phase were limited to acidic or neutral.
Meanwhile, porous-polymer packing material features a wide pH range and high sample loadability but it has the disadvantage of low separation efficiency, low pressure resistance, and limitation for organic content because of polymer's inherent swelling and contracting nature, and the column cost is high. Polymer gels are now being developed to improve the pressure resistance and to reduce the swelling and contraction rate by raising the degree of crosslinking.
Under these circumstances, Osaka Soda developed a silica packing material with basic resistance and improved durability as well as excellent pressure resistance, chemical stability, and separation performance over conventional ODS silica.
|
| Structure |
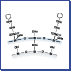
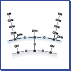
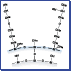
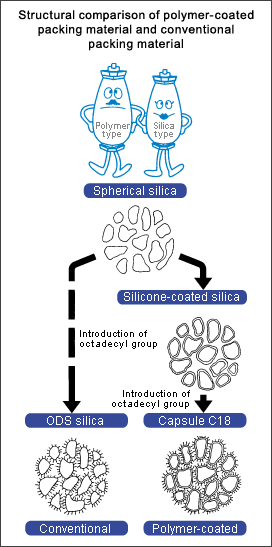
Figure 1 illustrates the structural differences between capsule-type packing material and the conventional chemically bonded packing material.
Conventional chemically bonded packing material is created by directly bonding alkyl groups to silanol groups on the surface of silica support using a silane coupling agent. Capsule-type packing material is created by covering silica support with a uniform thin film of silicone polymer and then bonding alkyl groups.
Figure 2 shows the bonding of each functional group to silica in CAPCELL PAK.
|
【Fig. 1】 Conceptual drawing of ODS silica and capsule-type C18
|
|
| Film thickness and pore |
Figure 3 shows the transition of pore distribution during the process of silica ? silicone polymer coated silica ? capsule-type C18 packing material.
The silica is spherical particles, 5 µm in diameter. The average pore size is about 6 nm in radius and 12 nm in diameter. Silicone polymer coating reduced the pore diameter by about 0.7 nm in radius (coating thickness: about 0.7 nm). Since C18 reaction also bonded C18 groups inside the pores, the pore radius was further reduced by 0.8 nm.
The two-step reaction reduced the pore size at each step but did not change the pore distribution width. This indicates uniform silicone resin coating and uniform introduction of C18 groups. |
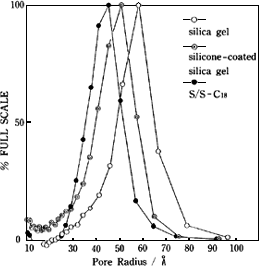 【Fig. 3】 Pore distribution of silica, silicone-coated silica, and capsule-type C18 |
| Quality control |
The silica is inspected for particle size, pore size, specific surface area, pore volume, and metal content according to the standard values for thorough quality control at each manufacturing process.
Most CAPCELL PAK columns are classified not only by application based on the Pharmaceutical Affairs Law of Japan but also by USP category from the United States Pharmacopeia. These columns support worldwide applications.
Columns of stable quality are available at any time anywhere in the world and can be used for routine analysis.
|